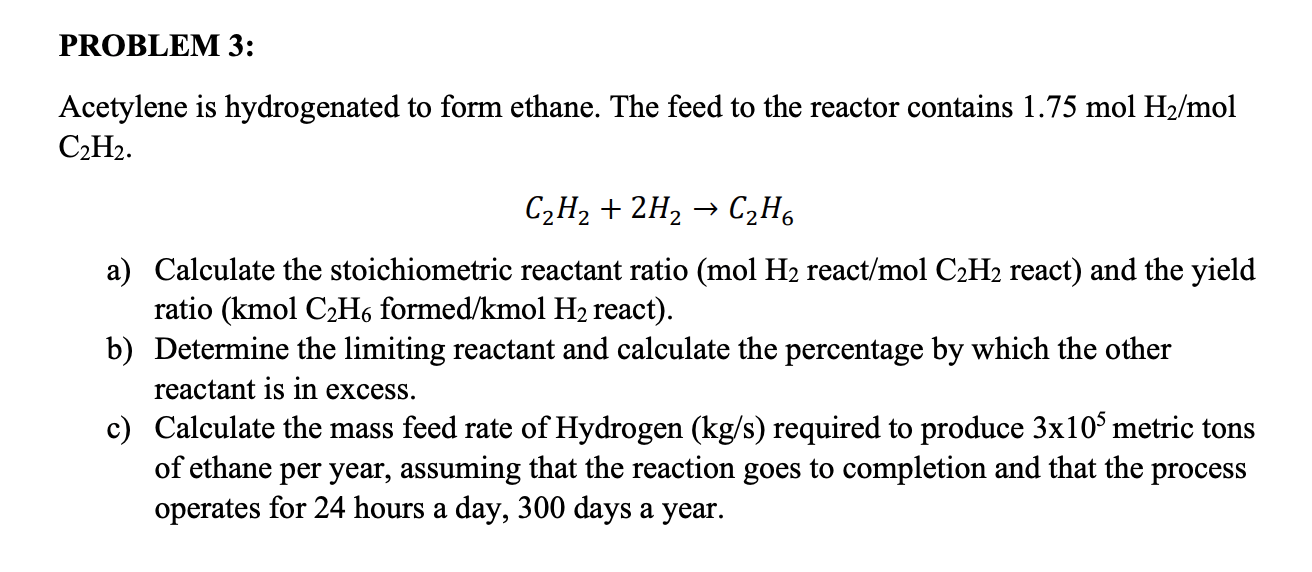
We have the balanced reaction as follows: A) write the balanced chemical reaction for this process. 0.7 mol h2 reacted/mol c2h2 reacted 0.5 mol c2hs formed/mol h2 reacted b. Web acetylene is hydrogenated to form ethane in the following reaction c2h2 + 2h2 → c2h6. Find all video solutions for your textbook.
The feed to the reactor contains 1.50molh2/molc2h2 1.50 m o l h 2 / m o l c 2 h 2. Web four types of behavior have been distinguished: The feed to the reactor contains 1.50 mol h2/mol c2h2. (a) calculate the stoichiometric reactant ratio (mol h2 react/mol c2h2 react) and the stoichiometric yield ratio (kmol c2h6 formed/kmol h2 react).
Web the limiting reactant in the hydrogenation of acetylene to ethane, with feed containing 1.4 mol h2/mol c2h2, is acetylene (c2h2). It is deficient by 30%. The feed to the reactor contains 1.50 mol h2/mol c2h2.
Acetylene Formula Preparation, Properties, and Uses What's Insight
The feed to the reactor contains 1.50 \mathrm {mol} \mathrm {h}_ {2} / \mathrm {mol} 1.50molh2/mol \mathrm {c}_ {2} \mathrm {h}_ {2} c2h2. Calculate the stoichiometric reactant ratio (mol. (a) calculate the stoichiometric reactant ratio (mol h2 h 2 react/mol c2h2 c 2 h 2 react) and the yield ratio (kmol c2h6 c 2 h 6 formed/kmol h2 h 2 react. C2h2 + 2h2 → c2h6 (a) stoichiometric ratio mol h2 react/mol c2h2 react = 2 mol h2 / 1 mol c2h2 yield ratio kmol c2h6formed/kmol h2 react = 1 kmol c2h6 / 2 kmol h2 (b) we have 1.50 mol h2/mol c2h2 ie. Web hydrogenation of gas mixtures enriched in acetylene and hydrogen in which acetylene is not an impurity, but the main component that needs to be quantitatively converted into ethylene, is also of great scientific and practical interest.
Web acetylene is hyrodgenated to form ethane. The reaction proceeds to completion a. (marks 20) acetylene (c2h2) is hydrogenated to form ethane (c2h6).
The Feed To The Reactor Contains 1.50Molh2/Molc2H2 1.50 M O L H 2 / M O L C 2 H 2.
Web acetylene is hyrodgenated to form ethane. The feed to the reactor contains 2.25 moles of h_2 for every mole of c_2h_2. Calculate the stoichiometric reactant ratio (mol h2 reacted/mol c2h2 reacted) and the yield ratio (mol czhs formed/mol h2 reacted). Write the stoichiometric equation for this reaction, and then answer the following questions:
Calculate The Stoichiometric Reactant Ratio (Mol.
Web acetylene is hydrogenated to form ethane. (a) calculate the stoichiometric reactant ratio (mol h2 h 2 react/mol c2h2 c 2 h 2 react) and the yield ratio (kmol c2h6 c 2 h 6 formed/kmol h2 h 2 react. A) write the balanced chemical reaction for this process. The reaction proceeds to completion a.
Web Acetylene Is Hydrogenated To Form Ethane.
Web four types of behavior have been distinguished: Calculate the stoichiometric reactant ratio (mol h2. 0.7 mol h2 reacted/mol c2h2 reacted 0.5 mol c2hs formed/mol h2 reacted b. The feed to the reactor contains 1.50 mol h2/mol c2h2.
It Is Deficient By 30%.
Web hydrogenation of gas mixtures enriched in acetylene and hydrogen in which acetylene is not an impurity, but the main component that needs to be quantitatively converted into ethylene, is also of great scientific and practical interest. In the ethane molecule, the bonding picture according to valence orbital theory is very similar to that of methane. The feed to the reactor contains 1.40 mol h2/mol c2h2. The feed to the reactor contains 1.50 mol h2 /mol c2h2.
Web acetylene is hydrogenated to form ethane in the following reaction c2h2 + 2h2 → c2h6. The feed to thereactor contains 1.5 mol h2/mol c2h2.a. The reaction proceeds to completion. Acetylene is hydrogenated to form ethane (c2h6). Web write the balanced equation for the reaction of acetylene and hydrogen to form ethane.