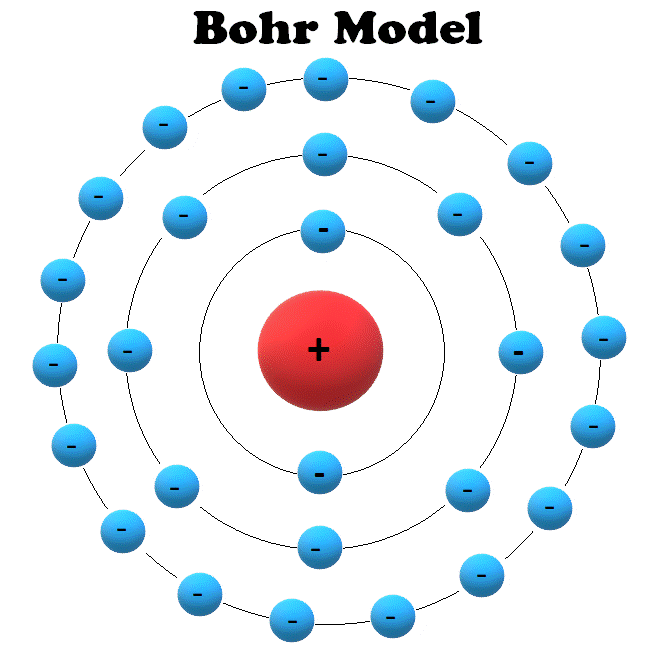
Draw the first electron shell and put the electrons as a dot in it. The energy states of the hydrogen atom. Find the number of protons, electrons, and neutrons of an atom. We’ll use a bohr diagram to visually represent where the electrons a. Begin by sketching the nucleus, and then draw the four electron shells.
Web do you want to learn how to build an atom from scratch? Web steps to draw bohr model of boron. It does introduce several important features of all models used to describe the distribution of electrons in an atom. • the atomic number of boron is 5.
The nucleus is shown as one green circle in the center. 1.3k views 1 year ago home school chemistry. You can also play a fun game to check your understanding of atomic concepts.
This simulation is part of the phet project, a leading provider of free online stem resources. Web as we now know the values of both the nuclear species i.e. Web quantum mechanics bohr equation. 4.5k views 2 years ago atomic structure. E ( n) = − 1 n 2 ⋅ 13.6 ev.
Find the number of protons, electrons, and neutrons of an atom. 4.5k views 2 years ago atomic structure. The boron family is located in the 13 th group of the periodic table:
Postulates Of Bohr’s Model Of An Atom.
Protons and neutrons, we can draw the nucleus of the bohr model of the oxygen atom. Web this chemistry tutorial video walks you through how to draw a bohr model, bohr diagram, or planetary model for an atom or ion. 48k views 6 years ago science 10. In the next step, we will calculate the total number of electrons contained in the oxygen atom.
Begin By Sketching The Nucleus, And Then Draw The Four Electron Shells.
The boron family is located in the 13 th group of the periodic table: Draw the nucleus of an atom. The charge, as well as the number of particles, is also specified in the model. Information derived from the boron box:
The Nucleus Is Shown As One Green Circle In The Center.
Web to draw the bohr model of an atom, follow these basic steps. It does introduce several important features of all models used to describe the distribution of electrons in an atom. Intro to general chemistry 3h 51m. Define an energy level in terms of the bohr model.
Home School Chemistry Day 15 Unit 2:
1.3k views 1 year ago home school chemistry. Web bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the danish physicist niels bohr. These orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the various shells. Write the number of protons and neutrons at the center of the nucleus.
Protons and neutrons, we can draw the nucleus of the bohr model of the oxygen atom. Bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. To understand this model better, we first need to understand the terminology used in this model for explaining the atomic structure. In this model, the electrons are represented as black dots that sit on a ring around the nucleus. The boron atom along with its atomic number, electronic configuration, and atomic mass is represented as follows in the periodic table: