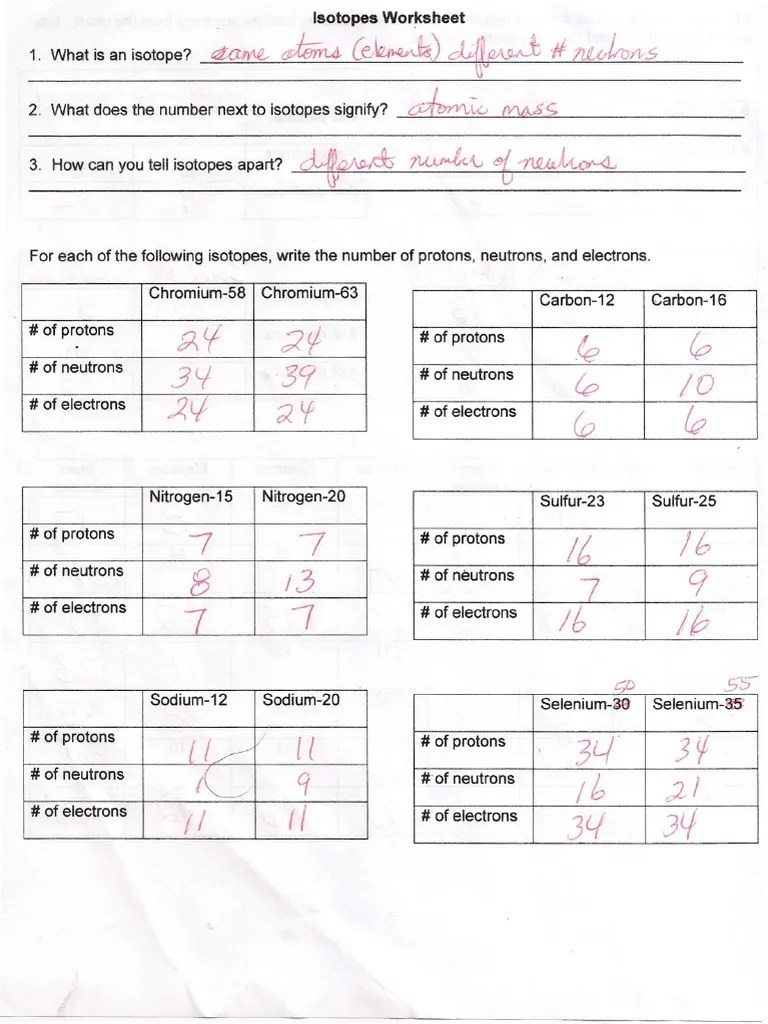
The number of protons in an atom is known as the of that plus the atom. Fill in the isotope names and any missing information, including isotope numbers from the chart. Label the atomic mass and atomic number. Web be able to write the standard nuclide notation for an isotope; The number of determines the name of the atom.
6 protons & 6 neutrons e. Chemistry (2012097) find the parts of an atoms given isotope information. They have different #s of neutrons; Use the element in your bock as an example.
Use the element in your bock as an example. Web an isotope is a variant of an element in which it has an equal number or protons but a varied number of neutrons. What part of the atom contains practically all its mass?
Isotope Notation Chemistry Worksheet 20 Problems With Answers Made By
The number of determines the name of the atom. Identify which among the isotope symbols below is incorrect. 6 protons & 6 neutrons e. The numbers 12, 13, and 14 refer to the mass number d. Be able to categorize elements by position in the periodic table
The mass number of an atom is the number of plus the number of in the nucleus of the atom. They have different #s of neutrons; Use your periodic table and the information provided.
This Resource Is Suitable For High School Chemistry Students Or Higher Education Students Learning About Isotopes.
What part of the atom contains practically all its mass? Included in the chemistry instructor resources subscription. The notation of an isotope occurs by adding a subscipt and superscript to the left side of an element such as 238 92u (uranium isotope) Be able to determine the numbers of fundamental particles in atoms and ions;
Web This Is A Worksheet Of Extra Practice Problems For Students Who Struggled With The Ions And Ion Notation Worksheet, And/Or The Isotopes And Isotope Notation Worksheet.
What is the isotope notation of the element that has an atomic number of 24 and a mass number of 52. Explain what each one tells you. Be able to categorize elements by position in the periodic table Write a nuclide symbol to represent this isotope.
How Are They The Same?
The mass number of an atom is the number of plus the number of in the nucleus of the atom. Be able to calculate atomic mass of a mixture of isotopes and percent isotopic composition; The number of determines the name of the atom. How many protons and neutrons are in the first isotope?
Isotopes Are Nearly Identical And Have The Same Number Of Protons, E.g.
6 12c 6 13c 6 14c. The numbers 12, 13, and 14 refer to the mass number d. 6 protons & 6 neutrons e. Isotopes, isotope notation, neutrons, atomic mass.
Web an isotope is a variant of an element in which it has an equal number or protons but a varied number of neutrons. Select your preferences below and click 'start' to give it a try! How many protons and neutrons are in the second isotope? Be able to categorize elements by position in the periodic table _______________________ sketch a block of any element on the periodic table.