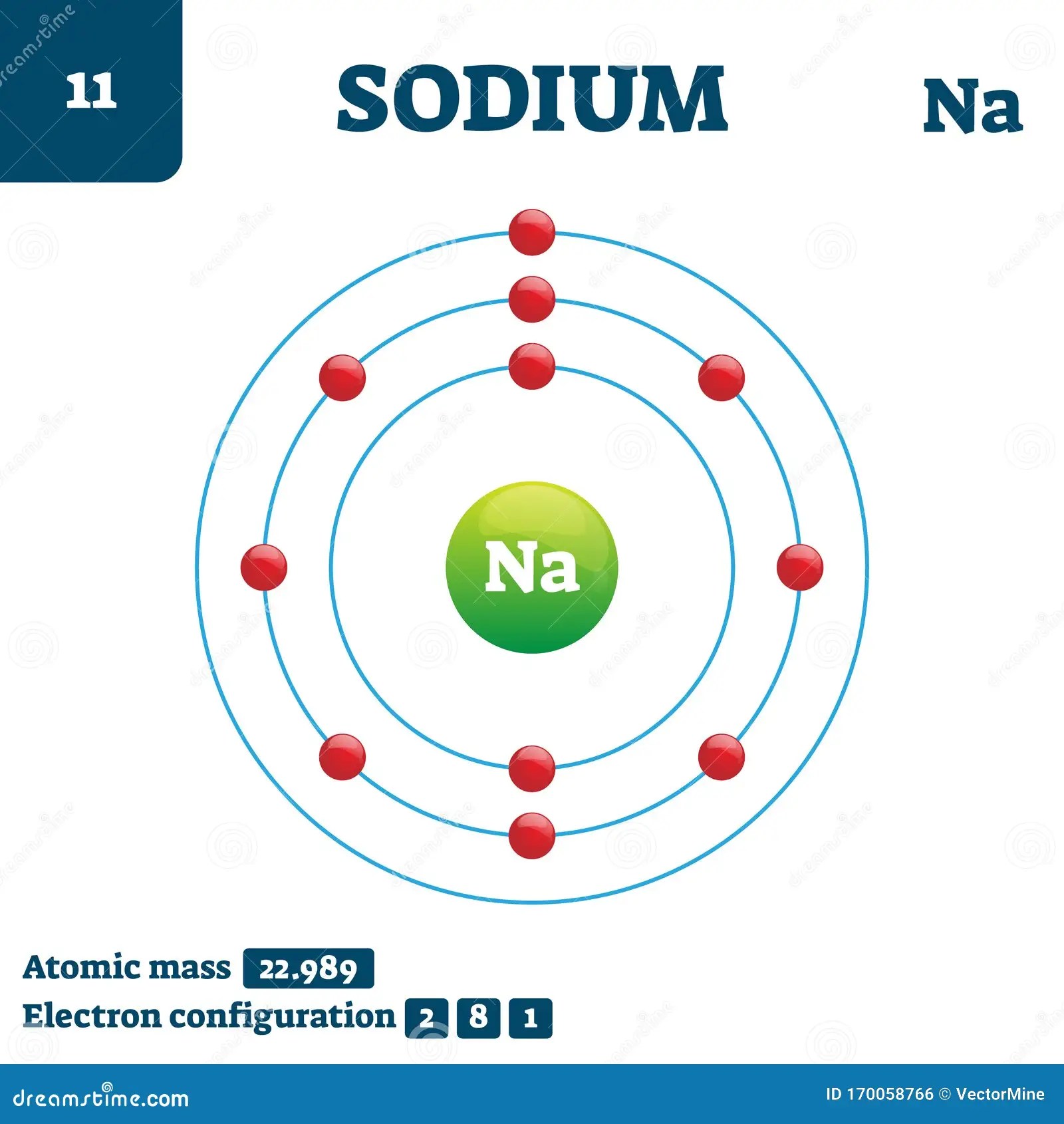
This leads to the frequent consumption of sodium beyond physiological need, the adverse health implications of which are generally accepted. The most common sodium compound is sodium chloride, or table salt. In contrast to many minerals, sodium absorption in the small intestine is extremely efficient and in a healthy individual all excess sodium is excreted by the kidneys. Sodium is a reactive alkali metal and is much more stable in ionic compounds. Reaction with air, water, and hydrogen.
In contrast to many minerals, sodium absorption in the small intestine is extremely efficient and in a healthy individual all excess sodium is excreted by the kidneys. The reason is that sodium attaches itself very strongly to other elements. In contrast to many minerals, sodium absorption in the small intestine is extremely efficient and in a healthy individual all excess sodium is excreted by the kidneys. Web sodium (na) is the sixth most abundant element on earth.
The majority of the sodium in the body is located in blood and in the fluid around cells. Web sodium has a brinell of 0.69 mpa, and mohs hardness of 0.5 mpa. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images.
Sodium is vital not only for maintaining fluid balance but also for many other essential functions. Its chemistry is well explored. In contrast to many minerals, sodium absorption in the small intestine is extremely efficient and in a healthy individual all excess sodium is excreted by the kidneys. Compounds of sodium have been known, of course, throughout human history. Sodium is the most common alkali metal and the sixth most abundant element on earth, comprising 2.8 percent of earth’s crust.
Electrolytes are salts and minerals, such as sodium, potassium, chloride and bicarbonate, which are found in the blood. Sodium is an alkali metal, being in group 1 of the periodic table. Web sodium is a mineral and a key component of salt.
It Accounts For 0.15% Of Your Body.
Family group 1 (ia) alkali metal. In contrast to many minerals, sodium absorption in the small intestine is extremely efficient and in a healthy individual all excess sodium is excreted by the kidneys. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. In contrast to many minerals, sodium absorption in the small intestine is extremely efficient and in a healthy individual, all excess sodium is excreted by the kidneys.
Sodium Is The Ninth Most Abundant Element In The Human Body.
Web sodium is the sixth most abundant elements on earth. Salt is actually called sodium chloride because it is made up of 40% sodium and 60% chloride. Web sodium is easily attainable within the human diet and we have developed a sodium preference and consequent tendency to consume sodium even when we are replete. They can conduct electrical impulses in the body.
The Young Modulus Of Sodium (Na) Is 10 Gpa.
Web sodium has a brinell of 0.69 mpa, and mohs hardness of 0.5 mpa. In contrast to many minerals, sodium absorption in the small intestine is extremely efficient and in a healthy individual all excess sodium is excreted by the kidneys. According to a 2019 study published in nutrients, sodium plays a crucial role in maintaining healthy blood pressure levels, as it helps to. To relieve stress and strains of daily life.
The Reason Is That Sodium Attaches Itself Very Strongly To Other Elements.
Body sodium (na) levels must be maintained within a narrow range for the correct functioning of the organism (na homeostasis). It can also form intermetallic compounds and organosodium compounds. This leads to the frequent consumption of sodium beyond physiological need, the adverse health implications of which are generally accepted. The speed of sound of sodium is 3200 m/s.
Web sodium is vital not only for maintaining fluid balance but also for many other essential functions. This leads to the frequent consumption of sodium beyond physiological need, the adverse health implications of which are generally accepted. Web sodium is the sixth most abundant elements on earth. The majority of the sodium in the body is located in blood and in the fluid around cells. But sodium metal was not prepared until 1807.



/GettyImages-sb10067655by-001-1e3eb6ce621f4747b863b631b5cad181.jpg)