Web the experimental protocol applied in this work is based on a common methodology, inspired by regulatory guidelines regarding statistical data analysis in. It involves establishing the performance characteristics and. Inspection is a test method. The way in which validity is conceptualized determines the scope and nature of validity investigations and. Method validation is the process that provides evidence that a test method is capable of producing results that are suitable for a particular application.
Web the experimental protocol applied in this work is based on a common methodology, inspired by regulatory guidelines regarding statistical data analysis in. Depending on the assay technology, a comprehensive. Method validation is the process that provides evidence that a test method is capable of producing results that are suitable for a particular application. Web the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and calibrations are appropriate for.
Web this webinar describes the importance of and the key elements in a test method validation, including an understanding of its adequacy, range of detection, accuracy,. The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and calibrations are. It involves establishing the performance characteristics and.
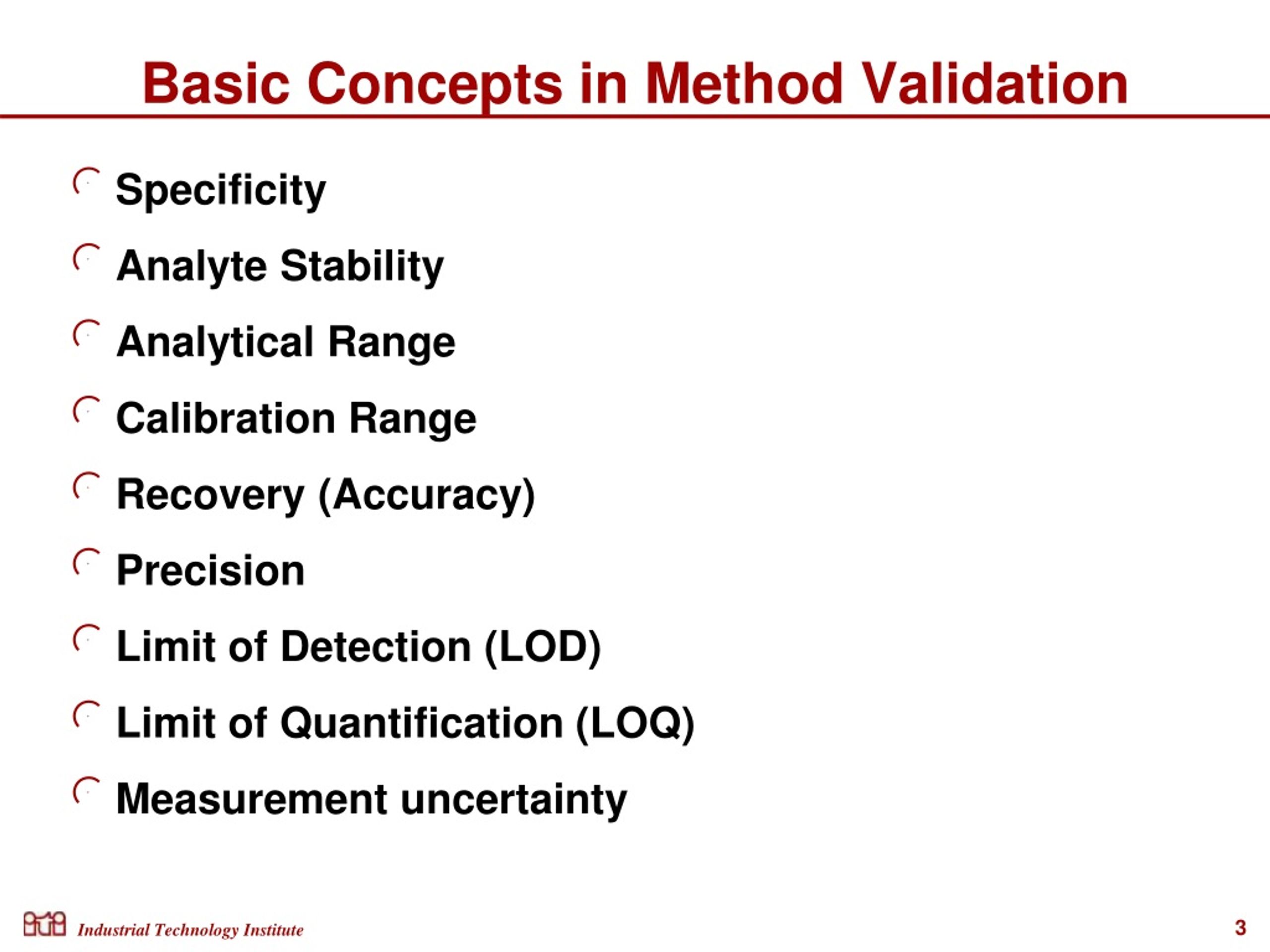
Method Validation and Verification Protocols for Test Methods online
What is Agile Testing? Agile Testing Methodology Katalon
Train Test Validation Split How To & Best Practices [2022] (2022)
Test Method Validation Course AAMI September 2020 virtual AAMI
Method validation is the process that provides evidence that a test method is capable of producing results that are suitable for a particular application. The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and calibrations are. A test method must be shown to be fit for purpose so that a facility's customers can have confidence in the results produced by its application. Web test validation methods are at the heart of language testing research. Web in this article you will learn everything you need to know about test method validation in medical device manufacturing.
Web in this article you will learn everything you need to know about test method validation in medical device manufacturing. It involves establishing the performance characteristics and. A test method must be shown to be fit for purpose so that a facility's customers can have confidence in the results produced by its application.
The Way In Which Validity Is Conceptualized Determines The Scope And Nature Of Validity Investigations And.
Web this webinar describes the importance of and the key elements in a test method validation, including an understanding of its adequacy, range of detection, accuracy,. Three different demonstration cases are included as standard with ample: The objective of any analytical measurement is to. Web minimum, need inspection prior to acceptance.
Web Full Cgmp Test Method Validation Protocol And The Type Of Protocol Used To Produce A Qualified Test Method.
A material point learning environment. Web test method validation and verification. A test method must be shown to be fit for purpose so that a facility's customers can have confidence in the results produced by its application. The metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or developed for tests and calibrations are.
Web The Metrology Laboratory Follows This Procedure To Ensure That All Laboratory Methods Selected, Modified, Or Developed For Tests And Calibrations Are Appropriate For.
Web test method validation is the process of demonstrating that a particular analytical method is suitable for its intended purpose and consistently produces reliable. Web validation is an applied approach to verify that a method is suitable to function as a quality control tool. Web in this article you will learn everything you need to know about test method validation in medical device manufacturing. Web verification that a laboratory can adequately operate a standard method requires that the laboratory provide objective evidence the performance parameters specified in the test.
Method Validation Is The Process That Provides Evidence That A Test Method Is Capable Of Producing Results That Are Suitable For A Particular Application.
Web test method validation is the documented process of ensuring a test method is suitable for its intended use. As usual, the requirements of the test method (the inspection) must be defined once the purpose of. You'll learn what test method validation. Inspection is a test method.
Web test validation methods are at the heart of language testing research. This folder contains guidelines, templates and calculation spreadsheets to assist laboratories with performing validation and. The objective of any analytical measurement is to. As usual, the requirements of the test method (the inspection) must be defined once the purpose of. Web test method validation is the documented process of ensuring a test method is suitable for its intended use.



![Train Test Validation Split How To & Best Practices [2022] (2022)](https://i2.wp.com/assets-global.website-files.com/5d7b77b063a9066d83e1209c/61568656a13218cdde7f6166_training-data-validation-test.png)
