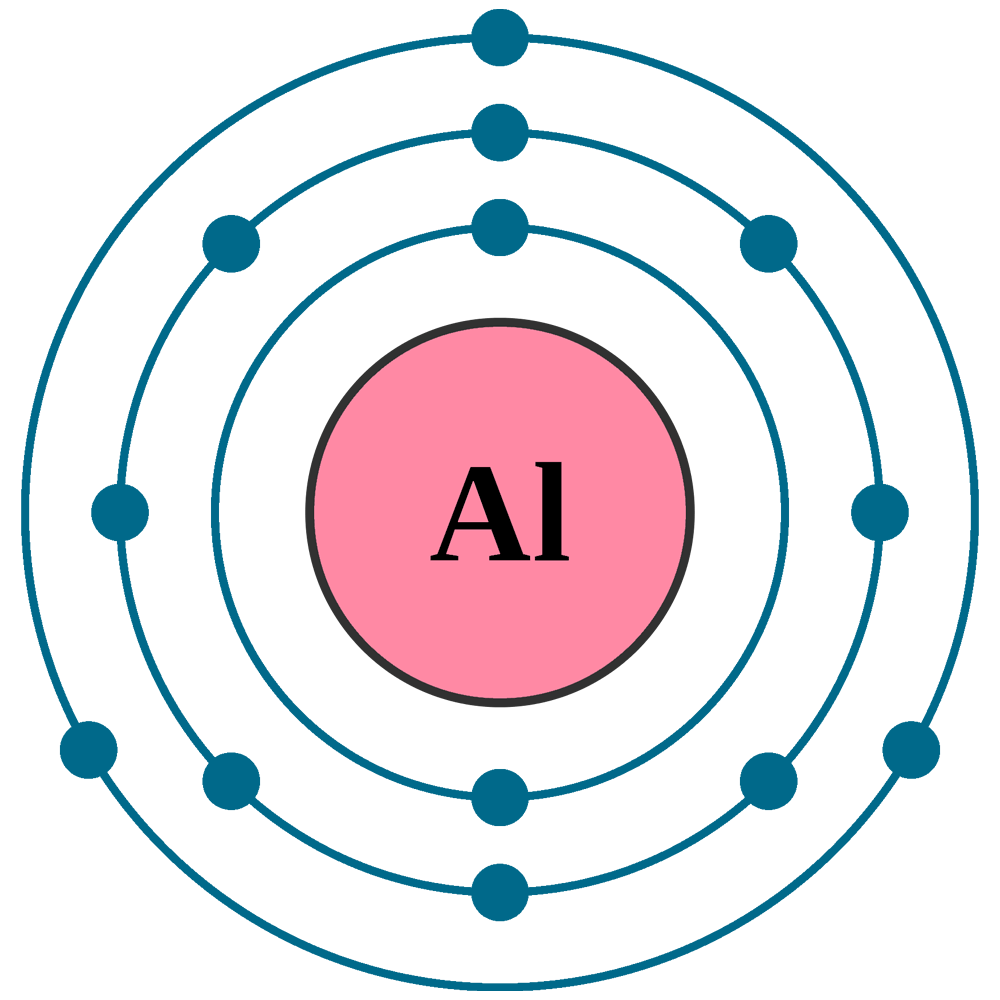
Web chemistry for allied health (soult) 2: Web aluminum ion(al 3+) electron configuration. The ground state electron configuration of aluminum is 1s 2 2s 2 2p 6 3s 2 3p 1. Write the symbol for each ion and name them. As mentioned above, there are positive and negative ions.
Transition and inner transition metal elements behave differently than main group elements. Web to find the electron configuration of an atom, you first need to know the number of electrons that it has. Its electronic configuration is 1s²2s²2p⁶3s²3p¹. Web because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13.
Define ion, cation, and anion. As mentioned above, there are positive and negative ions. Since aluminum's atomic number is thirteen, it has thirteen electrons.
Therefore, an aluminum atom has fourteen neutrons. Its electronic configuration is 1s²2s²2p⁶3s²3p¹. The aluminum atom_loses_____electrons to form an ion. In general, the cation name is the same as the element name, but with the word ion added to the end. A proton never moves from one atom to another.
Write the symbol for each ion and name them. Aluminium has the electronic configuration as 1s² 2s² 2p⁶ 3s² 3p¹. Recognize characteristics of monatomic and polyatomic ions.
( 20 +) + ( 18 −) = 2 +.
Web because the number of protons remains unchanged when an atom forms an ion, the atomic number of the element must be 13. Web on the right, the chloride ion has 18 electrons and has a 1− charge. As always, there are exceptions. Recognize characteristics of monatomic and polyatomic ions.
Predicting Charges On Monatomic Cations And Anions.
Therefore, the valency and valence electrons of aluminum are 3. After the electron configuration, the last shell of the aluminum atom has three electrons. In this video we’ll use the periodic table and a few simple rules to find the number of protons and electrons for the. 20 × ( 1 +) = 20 + charge from electrons:
Aluminum Is A Metal And It Is Located In The Third Group Or The 13Th Column Of The Periodic Table Of Elements.
In table salt, this electron comes from the sodium atom. If we want to summarize the process of chlorine gaining an electron to form the ion, we may write it like this: C 4−, a carbide ion. How do we name main group cations?
It Is Important To Note, However, That The Formula For An Ionic Compound Does Not Represent The Physical Arrangement Of Its Ions.
Explain how and why cations and anions are formed. Al 3 +, an aluminum ion. 18 × ( 1 −) = 18 − net charge: 31k views 3 years ago.
A proton never moves from one atom to another. Individual atoms can gain or lose electrons. Web the energy released when an electron is added to the neutral atom and a negative ion is formed. Explain how and why cations and anions are formed. For example, the alkali metals in group 1 on the periodic table tend to form cations with a 1 + charge.

:max_bytes(150000):strip_icc()/aluminiumatom-58b602655f9b5860464c6f7d.jpg)



