You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Covalent and ionic bonds are intramolecular forces. Web which of the following compounds can form intermolecular hydrogen bonds with a molecule identical to itself (electron pairs are not drawn in)? Web hydrogen bonding is the strongest type of intermolecular bond. Hydrogen bonds are intermolecular forces;
Which of the following compounds will form intermolecular hydrogen bonds in the liquid state? The evidence for hydrogen bonding. Web a hydrogen bond is an intermolecular attractive force in which a hydrogen atom that is covalently bonded to a small, highly electronegative atom is attracted to a lone pair of electrons on an atom in a neighboring molecule. Intermolecular hydrogen bonds occur between separate molecules in a substance.
The atom that loses an electron becomes a positive ion. Web intermolecular hydrogen bonds. World of chemistry, 3rd edition.
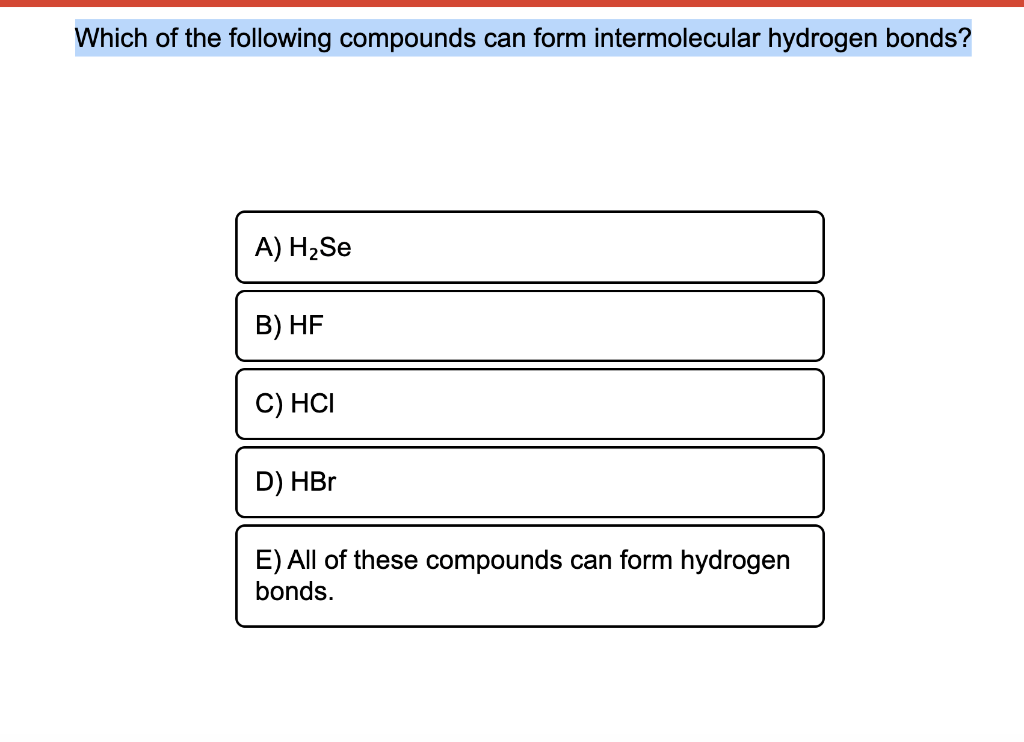
There are exactly the right numbers of δ+ hydrogens and lone pairs so that every one of them can be involved in hydrogen bonding. Many elements form compounds with hydrogen. Hydrogen forms polar covalent bonds to more electronegative atoms such as oxygen, and because a hydrogen atom is quite small, the positive end of the bond dipole (the hydrogen) can approach neighboring nucleophilic. A) h2se b) hf c) hci d) hbr. In order to mix the two, the hydrogen bonds between water molecules and the hydrogen bonds between ethanol molecules must be broken.
They can occur between any number of like or unlike molecules as long as hydrogen donors and acceptors are present in positions where they can interact with one another. Which of the following compounds can form intermolecular hydrogen bonds? Intermolecular hydrogen bonds occur between separate molecules in a substance.
(Select All That Apply.) Ch4.
A) h se b) hf c) hci d) hbo e) all of these compounds can form hydrogen bonds. The atom that loses an electron becomes a positive ion. World of chemistry, 3rd edition. Hydrogen bonds are intermolecular forces;
Here’s The Best Way To Solve It.
Web ch4 hf br2 ch3cooh. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. When a hydrogen atom is bonded to a highly electronegative atom hydrogen bonding occurs. Web type of compound intermolecular forces present relative order of boiling and melting points;
E) All Of These Compounds Can Form Hydrogen Bonds.
Web which of the following molecules can form hydrogen bonds with other molecules of the same kind: They can occur between any number of like or unlike molecules as long as hydrogen donors and acceptors are present in positions where they can interact with one another. Hydrogen forms polar covalent bonds to more electronegative atoms such as oxygen, and because a hydrogen atom is quite small, the positive end of the bond dipole (the hydrogen) can approach neighboring nucleophilic. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.
Hydrogen Bond Is An Intermolecular Force (Imf) That Fo.
Web in both pure water and pure ethanol the main intermolecular attractions are hydrogen bonds. Web which of the following compounds can form intermolecular hydrogen bonds with a molecule identical to itself (electron pairs are not drawn in)? The evidence for hydrogen bonding. 100% (4 ratings) share share.
Web water as a perfect example of hydrogen bonding. This problem has been solved! Web a hydrogen bond is an intermolecular attractive force in which a hydrogen atom that is covalently bonded to a small, highly electronegative atom is attracted to a lone pair of electrons on an atom in a neighboring molecule. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Which of the following compounds can form intermolecular hydrogen bonds?